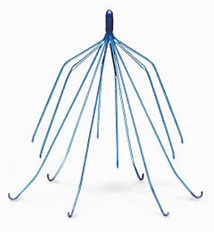
If there was ever a medical device that was rushed to market and continued to be marketed after the company knew that the device was defective, look no further. Bard and other Inferior Vena Cava filter device makers are under fire for selling a grossly defective medical device that is responsible for at least 27 deaths with the Bard Recovery filter over the past 10 years.
South Dakota Bard IVC Filter Injury
Amber Nordin, from South Dakota earlier this year filed a personal injury and defective product liability lawsuit against C.R. Bard, because of the company’s Eclipse blood clot filters broke apart and embedded inside her internal organs.
According to Nordin who filed her case in the U.S. District Court for the District of South Dakota, she received a Bard Eclipse IVC filter in October 2012. She then discovered that the medical device had migrated out of position and became lodged in her organs.
The Bard Eclipse medical device is designed to be implanted in the inferior vena cava (IVC) for patients at increased risk of a pulmonary embolism or a blood clot to the lung. The device and several other retrievable filter designs sold by Bard are associated with catastrophic and deadly injuries for thousands of patients.
Nordin alleges that after the Bard Eclipse IVC filter embedded in her internal organs, she had unsuccessful surgery to remove the blood clot filter in January 2014, but it remains imbedded inside her body.
Nordin will face more problems from the Bard IVC filter in the future, including life-threatening complications and ongoing medical care to ensure the Bard Eclipse filter does not cause further injury.
The case joins a number of similar Bard IVC filter lawsuits filed in recent years, alleging that the devices are unreasonably dangerous and defective. In addition to the Eclipse, similar problems have been reported involving the Bard G2, Bard Recovery and Bard Meridian filter designs.
What is the problem with IVC Filters?
The IVC filters are retrievable devices that are intended to be removed once a patient is no longer at risk for pulmonary embolism. A study in JAMA: Internal Medicine in April 2013 found that successful IVC retrieval occurred in less than 10% of patients.
In 2010, the U.S. Food & Drug Administration (FDA) disclosed that retrievable IVC filters had been associated with more than 900 adverse events, including incidents of migration, fracture and embolization, and perforation of the inferior vena cava. In 2014, the agency issued a new advisory to remind doctors that retrievable IVC filters should be removed within 29 to 54 days of their implantation.
Federal Court Multidistrict Litigation
There are two major manufacturers of retrievable IVC filters, C.R. Bard and Cook. The U.S. Judicial Panel on Multidistrict Litigation (JPML) has granted coordinated pretrial proceedings in the federal court system, centralizing all Bard IVC filter injury lawsuits before U.S. District Judge David G. Campbell in the District of Arizona.
According to the JPML data as of February 16, there are about 85 cases involving C.R. Bard’s Recovery and G2 IVC filter pending in the District of Arizona.
There are more than 250 Cook IVC filter lawsuits involving the company’s Celect and Gunther Tulip blood clot filters that have been centralized in a multidistrict litigation in the U.S. District Court, Southern District of Indiana.
As is common in product liability MDL cases, a series of early trial dates known as “bellwether trials” will be scheduled against each of the IVC filter makers to assist the parties determine how juries may respond to testimony over the long-term risks associated with the IVC filters.
NBC News Investigation
In September of 2015, a NBC News investigation revealed that executives at C.R. Bard were well aware of the dangers of their original Recovery IVC filter. Instead of issuing a recall, however, the company simply came out with a slightly modified version, which they called the “G2.”
Despite hundreds of complaints to the FDA indicating that the G2 failed to correct the problems of the earlier model, Bard not only failed to correct the issues associated with the Recovery filter, but refused to recall the product.
(Cook Medical, Inc., IVC Filters Marketing, Sales Practices and Products Liability Litigation – MDL 2570; In Re: Bard IVC Filters Products Liability Litigation – MDL No. 2641).
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog