According to a recent ruling by the U.S. Judicial Panel on Multidistrict Litigation (JPML), all filed Bard Inferior Vena Cava (IVC) filter injury, perforation and migration lawsuits, are to be consolidated and centralized before one federal judge in Phoenix, Arizona for coordinated pretrial proceedings.
 Existing cases pending throughout the federal court system will be transferred to U.S. District Judge David G. Campbell, who will oversee the multidistrict litigation (MDL) proceedings. Bard Peripheral Vascular is the manufacturer of these Bard IVC filters and is headquartered in Phoenix, Arizona.
Existing cases pending throughout the federal court system will be transferred to U.S. District Judge David G. Campbell, who will oversee the multidistrict litigation (MDL) proceedings. Bard Peripheral Vascular is the manufacturer of these Bard IVC filters and is headquartered in Phoenix, Arizona.
This consolidation and centralization of Bard IVC filter cases only applies to cases filed in the federal court system. There are less than 50 filed Bard Recovery filter and Bard G2 filter lawsuits currently pending in U.S. District Courts nationwide.
Bard IVC Filter Injury Allegations
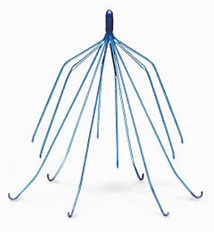
The lawsuits have similar allegations that plaintiffs experienced significant and serious side effects after having the blood clot filter medical devices implanted into the inferior vena cava.
The filter devices used when there is a blood clot in the leg known as a deep vein thrombosis and there is a contraindication from using blood thinners, which is the standard therapy for leg blood clots.
These devices are supposed to trap blood clots from traveling from the leg to the lungs and causing a pulmonary embolism, which can be fatal.
These devices unfortunately, have been linked to hundreds of adverse event reports where they puncture the vena cava, tilt out of position or migrate or break apart and cause metallic fragments to travel to the heart or lungs, a condition known as embolization.
Benefits of MDL Consolidation and Centralization
The MDL streamlines the trial process, by preventing duplicative discovery and all the plaintiffs and defendants are gathered before one judge.
MDL has been established for all Cook Medical IVC filter lawsuits, involving problems with the Cook Celect or Gunther Tulip filters. There are more than 100 complaints over those products centralized before U.S. District Judge Richard L. Young in the Southern District of Indiana.
MDL Bellwether IVC Filter Trials
It is common in complex multidistrict ligation involving pharmaceutical and medical device cases, to have mini trials, known as bellwether trials.
A small group of cases will be prepared for early trial dates. The outcome of these trials are designed to aid settlement negotiations, especially if the jury sides with the injured plaintiffs and awards a large damage award or in cases involving gross negligence, awarding punitive damages, which are designed to punish the company for its reckless behavior.
What is the Problem with IVC Filters?
The complaints in the Bard and Cook IVC Filter litigation have similar allegations, the injured patients claim that the manufacturers designed and sold retrievable defective devices that were unreasonably dangerous.
FDA Warnings
The FDA in August 2010, announced a warning about the risk of IVC filter injuries, noting that they had received hundreds of adverse event reports where filter devices broke apart and travelled to other areas of the body or caused perforation and migration.
Adverse Medical Studies
In March 2013, JAMA Internal Medicine journal questioned the effectiveness of IVC filters, noting that less than 10% of the study patients had successful removal of the devices and 8% of IVC filter patients developed pulmonary embolism despite the device.
In May 2014, the FDA cautioned doctors to remove IVC filters within about one to two months after the risk of a pulmonary embolism has passed, noting that many doctors were not adequately warned about the importance of retrieving the devices.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

