Power morcellators are medical devices that are used during laparoscopic hysterectomy and myomectomy procedures. The device allows the surgeon to grind up the uterus or uterine fibroids and remove the tissue through a small incision in the abdomen. The medical device is used during minimally invasive surgery, and reduces recovery time, scarring and speeds up recovery time.
Dr Shezad Malik Law Firm has offices based in Fort Worth and Dallas and represents people who have suffered catastrophic and serious personal injuries including wrongful death, caused by the negligence or recklessness of others. We specialize in Personal Injury trial litigation and focus our energy and efforts on those we represent.
Articles Posted in Personal Injury
Xarelto Louisiana MDL For Uncontrollable Bleeding Lawsuits
Over 50 Federal Xarelto Lawsuits
Bayer Healthcare and Johnson & Johnson’s Janssen Pharmaceuticals subsidiary are exposed to at least 50 product liability lawsuits over their new-generation anticoagulant. These personal injury and wrongful death claims are pending in 24 different federal courts.
Boston Scientific Ordered To Pay $26.7 M in Florida Transvaginal Mesh Cases
 Jurors in federal court in Miami found that Boston Scientific officials defectively designed their Pinnacle pelvic-organ implants and failed to properly warn doctors and their patients about the device’s risks.
Jurors in federal court in Miami found that Boston Scientific officials defectively designed their Pinnacle pelvic-organ implants and failed to properly warn doctors and their patients about the device’s risks.
The verdict is the first in a federal case against Boston Scientific over the Pinnacle inserts and the first to combine more than one plaintiff’s claims.
West Virginia Actos Bladder Cancer Trial
 The West Virginia trial comes shortly on the heels of a $2 million verdict in Philadelphia on October 3 over the blockbuster drug.
The West Virginia trial comes shortly on the heels of a $2 million verdict in Philadelphia on October 3 over the blockbuster drug.Takeda Knew in 2004 of Cancer Link
Jury Finds for J&J DePuy In Texas Pinnacle Hip Trial
The Dallas federal jury ruled unanimously against the plaintiff Kathleen Herlihy-Paoli, who said the two metal-on-metal Pinnacle hips she received in 2009 were defective and that the company failed to warn patients and doctors about the device’s risks.
Cook IVC Filter MDL Litigation Heading to Southern Indiana
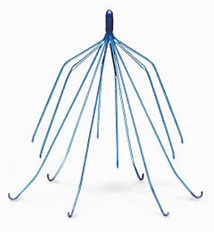 This order by the JPML establishes a nationwide federal Multidistrict Litigation (MDL) in one courthouse. This MDL will include all product liability and personal injury lawsuits involving side effect complications with Cook IVC filters.
This order by the JPML establishes a nationwide federal Multidistrict Litigation (MDL) in one courthouse. This MDL will include all product liability and personal injury lawsuits involving side effect complications with Cook IVC filters.
What are Cook IVC Filters?
Report: Cook IVC Filter Lawsuit MDL Centralization Hearing
The U.S. Judicial Panel on Multidistrict Litigation, earlier this week, considered the application for all Cook Celect and Gunther Tulip IVC filter lawsuits filed nationwide in U.S. District Courts. The application was brought by the plaintiffs and they requested that the litigation be centralized before one judge for coordinated pretrial proceedings, as part of an MDL, or Multidistrict Litigation.
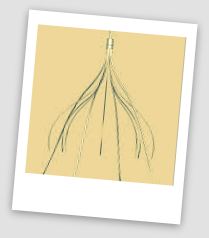 Cook Medical 27 IVC Filter Lawsuits
Cook Medical 27 IVC Filter Lawsuits
Currently, Cook Medical faced at least 27 different IVC filter lawsuits pending in 11 different districts. Plaintiffs have requested that the litigation be transferred to the U.S. District Court for the Southern District of Indiana as part of the MDL.
Report: DePuy Pinnacle First Texas Bellwether Trial
The first federal bellwether trial against Johnson & Johnson’s DePuy subsidiary for its allegedly defective Pinnacle Hip Implant has been underway for the past 3 weeks in Dallas, Texas.
The U.S. District Court for the Northern District of Texas oversaw jury selection on September 2 and over the past three weeks there has been a parade of company executives, paid doctor consultants and defense experts subjected to withering cross examination by the plaintiffs team of lawyers.
DePuy Orthopedics Inc., is facing the first bellwether trial over allegations that the Pinnacle metal-on-metal hip was defectively designed and caused metallosis. According to the plaintiff Kathleen Herlihy-Paoli, the cobalt-and-chromium alloy caused an infection that resulted in the artificial hips to be removed.
Cook Celect, Gunther Tulip IVC Filters Lawsuit Update
J&J Ethicon Hammered for $3.27M in Trans Vaginal Mesh Trial
A federal jury in West Virginia today hammered Johnson and Johnson’s Ethicon subsidiary over its transvaginal mesh device. The jury awarded $3.27 million to Jo Huskey over its defective Gynecare TVT Obturator, or TVT-O, transvaginal mesh device. This is one of 33,000 product liability injury lawsuits that claim Johnson & Johnson’s Ethicon Inc unit sold defective transvaginal mesh devices.
After a two-week trial, jurors found Ethicon liable for selling defective mesh devices and failing to warn patients and their doctors that they were at risk from serious side effects including pain, erosion, perforation and infection.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

