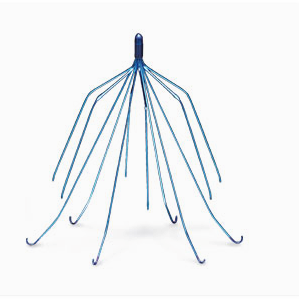
 Cook Medical, the makers of Gunther Tulip and other IVC filters, continue to face lawsuits from consumers who have accused the manufacturer of “selling dangerous medical devices and failing to warn about the risks” consumers face.
Cook Medical, the makers of Gunther Tulip and other IVC filters, continue to face lawsuits from consumers who have accused the manufacturer of “selling dangerous medical devices and failing to warn about the risks” consumers face.
Parker Thompson, a man from Texas who was implanted with the IVC filter in 2005, is the latest person to file a lawsuit against Cook Medical over their Gunther Tulip device. Thompson joins approximately 400 other people who are suing the Gunther Tulip IVC filter manufacturer in the U.S. District for the Southern District of Indiana.
The lawsuits allege that some of Cook’s blood-clot filters have broken apart, moved or poked through the blood vessel where they are implanted.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog


 More than a decade after the first injury linked to Takata’s defective air bags occurred, the largest recall in U.S. automobile history is underway. An estimated 100 million vehicles across the world are being recalled altogether.
More than a decade after the first injury linked to Takata’s defective air bags occurred, the largest recall in U.S. automobile history is underway. An estimated 100 million vehicles across the world are being recalled altogether. The largest recall in U.S. automotive history is on the way after federal safety regulators expanded a nationwide recall of Takata’s air bags to 69 million vehicles made by more than 14 automakers.
The largest recall in U.S. automotive history is on the way after federal safety regulators expanded a nationwide recall of Takata’s air bags to 69 million vehicles made by more than 14 automakers. Problems with transvaginal mesh systems marketed by a number of different medical device manufacturers have caused women throughout the United States to suffer severe injuries.
Problems with transvaginal mesh systems marketed by a number of different medical device manufacturers have caused women throughout the United States to suffer severe injuries.