Some patients who develop an infection after undergoing hip or knee replacement surgery have filed lawsuits against the makers of a device named Bair Hugger. The device is used in a number of hip and knee replacement surgery and may be linked to some serious side effects.
The Bair Hugger device is usually used without the patients knowledge. The device is a warming blanket used to help maintain a patient’s body temperature during surgery. Doctors utilize devices to maintain a patient’s body temperature because studies have shown that maintaining proper body temperature can reduce bleeding, speed up recovery time, and provide other benefits.
Surgical warming blankets, such as Bair Hugger, work like a forced-air heater, pushing warm air through a hose into a blanket covering a patient. The device releases warm air over a patient’s body, but also releases air under the surgical table. The air released under the table may have the potential to cause bacteria and germs to spread throughout the room, landing on a patient’s surgical site. This may cause an infection in the patient’s knee or hip, such as sepsis and Methicillin-resistant Staphylococcus aureus (MRSA). Unfortunately, these infections are very difficult to treat when they occur deep in a patient’s joint.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog



 According to a lawsuit filed by Ami Dodson, the drug caused her to suffer from permanent hair loss. Dodson’s lawsuit also alleges that the makers of Taxotere engaged in marketing schemes to drive up the sales of the drug while hiding the medication’s increased toxicity compared to other similar drugs.
According to a lawsuit filed by Ami Dodson, the drug caused her to suffer from permanent hair loss. Dodson’s lawsuit also alleges that the makers of Taxotere engaged in marketing schemes to drive up the sales of the drug while hiding the medication’s increased toxicity compared to other similar drugs.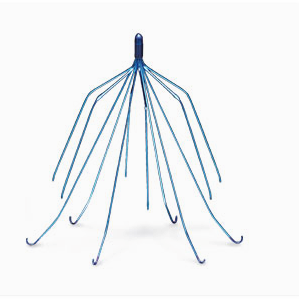 Five products produced by C.R. Bard and Cook Medical are at the center of these lawsuits. These products include:
Five products produced by C.R. Bard and Cook Medical are at the center of these lawsuits. These products include: Women in these lawsuits have claimed that after receiving the mesh implant, they have suffered injuries that include bleeding, infection, organ perforation, inability to engage in sexual intercourse, pelvic pain or severe vaginal pain, vaginal scarring, fistulas, erosion of mesh through the vagina, recurrent incontinence or prolapse, need for correctional surgery, urinary tract infections and neuromuscular problems. Spouses of some of the plaintiff have even included loss of consortium in their claims.
Women in these lawsuits have claimed that after receiving the mesh implant, they have suffered injuries that include bleeding, infection, organ perforation, inability to engage in sexual intercourse, pelvic pain or severe vaginal pain, vaginal scarring, fistulas, erosion of mesh through the vagina, recurrent incontinence or prolapse, need for correctional surgery, urinary tract infections and neuromuscular problems. Spouses of some of the plaintiff have even included loss of consortium in their claims.