More than 300 Lawsuits in Federal MDL
Dr Shezad Malik Law Firm has offices based in Fort Worth and Dallas and represents people who have suffered catastrophic and serious personal injuries including wrongful death, caused by the negligence or recklessness of others. We specialize in Personal Injury trial litigation and focus our energy and efforts on those we represent.
Articles Posted in Product Liability
Stryker Rejuvenate Hip Implants Ordered to Mediation
Stryker Rejuvenate hip lawsuits are on a fast track in New Jersey state court, with a court mandated mediation program in place to try and reach a settlement.
More than 430 defective hip product liability Stryker Rejuvenate and ABG II hip stems lawsuits are centralized in the Superior Court for Bergen County, New Jersey before Judge Brian R. Martinotti.
DePuy ASR Hip Failure Lawsuits Heading to Settlement?
There is media speculation that Johnson & Johnson (J&J), and its subsidiary DePuy, are in active discussions to attempt a global settlement of their defective ASR metal on metal artificial hips.
J&J Potential Settlement North of $3 Billion
Apparently J&J has discussed paying more than $3 billion to settle lawsuits over its recalled hip implants. J&J is exposed to over 11,500 product liability lawsuits in the U.S. and is considering paying more than $300,000 per case.
California DePuy ASR Hip Implant Failure Trial Settled
According to court documents, Johnson & Johnson has agreed to settle a DePuy ASR hip replacement lawsuit, on the eve of trial. The settlement agreement remains confidential.
Johnson & Johnson subsidiary DePuy Orthopaedics has agreed to settle a DePuy ASR hip lawsuit, the first bellwether trial in a California state court consolidation of ASR cases.
This DePuy ASR case that was set to begin later in October in California state court. See my earlier reporting on secret settlement negotiations regarding the DePuy ASR implants. This is overall good news for plaintiffs that the company is beginning to take the failure allegations seriously.
Bard, Boston Scientific, Endo Health/AMS Vaginal-Mesh Makers Settlement on the horizon
As a Texas Vaginal Mesh attorney, and Dallas medical doctor, we are reporting this important potential transvaginal mesh settlement breakthrough. According to sources, C.R. Bard Inc., and other makers of vaginal-mesh implants, are in advanced talks to settle thousands of vaginal mesh product liability lawsuits.
![]() Bard, Endo Health Solutions Inc. the parent of AMS, Boston Scientific Corp., Coloplast and Cook Medical Inc. manufacturers making vaginal mesh devices to treat pelvic organ prolapse (POP) and treat stress urinary incontinence (SUI) in talks about settling all suits.
Bard, Endo Health Solutions Inc. the parent of AMS, Boston Scientific Corp., Coloplast and Cook Medical Inc. manufacturers making vaginal mesh devices to treat pelvic organ prolapse (POP) and treat stress urinary incontinence (SUI) in talks about settling all suits.
Johnson & Johnson, which also faces suits over the vaginal mesh devices, isn’t involved in the talks.
Bard, Ethicon, Boston Scientific, AMS, Coloplast Vaginal Mesh Litigation
Transvaginal mesh injury lawsuits are consolidated and coordinated in 6 individual multidistrict litigations (MDLs) established in the federal court system. These injury claims against vaginal mesh manufacturers have now become the largest pending litigation in the U.S.
![]() 30,000 Active Mesh Lawsuits and Climbing
30,000 Active Mesh Lawsuits and Climbing
There are more than 30,000 complaints filed in U.S. District Courts over injuries with transvaginal mesh and bladder sling products. These medical devices have been used for treatment of pelvic organ prolapse (POP) and female stress urinary incontinence (SUI).
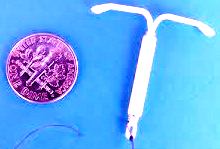
Mirena IUD Perforation Injury Lawsuits
As a Texas Mirena IUD Product Liability attorney, I am providing this timely Mirena litigation update. Mirena IUD product liability lawsuits continues to increase, with 400 complaints being filed in federal and state courts, across the country.
The U.S. Judicial Panel on Multidistrict Litigation (JPML) centralized all lawsuits filed in U.S. District Courts before one judge for coordinated pretrial proceedings.
Actos Trial: Takeda Duped Patients, Doctors on Actos Bladder Cancer Risk
According to the testimony presented in trial, Takeda Pharmaceutical Co. duped patients and doctors about the risks of its Actos diabetes drug and should be held liable for the cancer death of a former U.S. Army translator.
 Takeda did not disclose Bladder Cancer Risks
Takeda did not disclose Bladder Cancer Risks
The plaintiff alleged that Japan-based Takeda, failed to provide any warning until 2011 that researchers had linked Actos to bladder cancer. The family of Diep An, filed a wrongful death product liability lawsuit in state court in Baltimore, MD. The plaintiff’s attorney allege that the lack of a warning allowed the company to protect billions in sales of the drug.
Bard Avaulta, Ethicon Gynecare Vaginal Mesh for Prolapse Repair
As a Texas Transvaginal mesh attorney and medical doctor, I am providing details on this latest medical research. According to new published research, transvaginal mesh placement provides no benefits when used for pelvic organ prolapse repair. In a study published in the Obstetrics and Gynecology journal, researchers found that the use of vaginal mesh does not increase good outcome for surgical prolapse repair when compared to more traditional treatments.
 Georgetown University researchers halted the study due to the high rate of serious injuries associated with use of vaginal mesh. The study involved 65 women, 33 of whom received vaginal mesh to treat pelvic organ prolapse (POP) and 32 who underwent traditional vaginal prolapse surgery.
Georgetown University researchers halted the study due to the high rate of serious injuries associated with use of vaginal mesh. The study involved 65 women, 33 of whom received vaginal mesh to treat pelvic organ prolapse (POP) and 32 who underwent traditional vaginal prolapse surgery.
The women were followed for three years and the program stopped early because of a 15.6% mesh exposure rate, where the mesh eroded into the vagina.
J&J Will Stop Sales of Metal-on-Metal Hip Replacements
As a Texas Metal on Metal hip attorney and medical doctor I am providing this important update to the folks injured by a defective metal hip implant. According to Johnson & Johnson (JNJ), it will stop selling metal-on-metal and ceramic-on-metal hip replacements because of a lower demand for the devices.This decision announcement was made in May 2013.
Metal liners in the Ultamet Metal-on-Metal Articulation and the Complete Ceramic-on-Metal Acetabular Hip System will no longer be available worldwide after August 31. J&J’s DePuy Orthopaedics unit will stop selling related products through 2014 to simplify and streamline its offerings.
Metal on Metal Hip Replacement Sales
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog