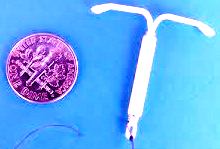
Many defective product liability and personal injury lawsuits have been filed over the Mirena intra uterine birth control device, in various State and Federal courts nationwide. Recently, a motion has been filed to consolidate all federal cases before one federal court for coordinated pretrial proceedings as part of an MDL, or multidistrict litigation.
Mirena IUD Perforation Allegations
All of the lawsuits involve similar complaints that the intra-uterine device (IUD), caused severe complications including perforating or puncturing the uterus, and migrating to other parts of the body. The motion to consolidate requested that all Mirena lawsuits be consolidated in the Northern District of Ohio.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog