 A device known as a vena cava filter (IVC filter) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. But recently two IVC filter devices named Cordis OptEase and Cordis TrapEase have came under scrutiny after lawsuits reveal potential design defects.
A device known as a vena cava filter (IVC filter) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. But recently two IVC filter devices named Cordis OptEase and Cordis TrapEase have came under scrutiny after lawsuits reveal potential design defects.
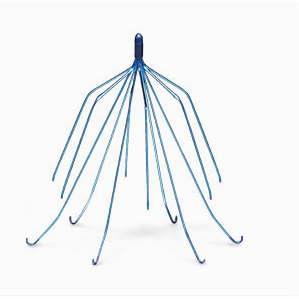
The Cordis Optease IVC filter is an egg-shaped wire device and Cordis TrapEase IVC filter is a star-shaped wire device. They are both implanted in a blood vessel called the inferior vena cava, which separates the heart and lungs. The filters are designed to catch blood clots before they enter the lungs and cause a pulmonary embolism.
The Cordis TrapEase IVC filter is supposed to be permanent, but studies have linked it to a 50% risk of fracture within four years. In another study, researchers warned about an “extremely high risk” of fracture within 2-3 years.
In November 2011, JAMA Internal Medicine published a study linking Cordis TrapEase with a 50% risk of fracture.
The conclusions were based on data from 20 people who were implanted with Cordis TrapEase between November 2002 and July 2006. After an average of 50 months (about four years), 10 filters (50%) were fractured.
Furthermore, about two-thirds of the filters were fractured after four years, suggesting the risk increases over time. Two patients suffered multiple fractures.
The researchers said patients “are at extremely high risk of strut fracture as early as 2 to 3 years” after the Cordis TrapEase is implanted. No life-threatening complications occurred in this study, but they have been reported in other studies.
Our law firm is currently investigating Cordis TrapEase and Optease IVC filter lawsuits from across the country. If you or a loved one suffered from an injury after having one of these IVC filters implated, contact our office immediately.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

