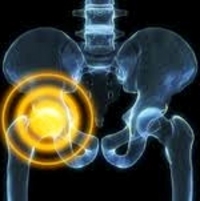
 Metal-on-metal hip implants have led to some patients developing metallosis, a condition in which build-up of metal debris in the soft tissues of the body occur.
Metal-on-metal hip implants have led to some patients developing metallosis, a condition in which build-up of metal debris in the soft tissues of the body occur.
Hip replacement surgery (hip arthroplasty) has been described by many experts as one of the most significant medical device innovations of the last 40 years. It has helped millions of people overcome painful arthritis, recover from hip fractures and improve their quality of life. However, hip implants do not come without risk or complications. A growing number of implant recipients have experienced implant failure and other severe hip replacement complications.
There are three types of hip replacement surgery. With total hip replacement, the entire hip joint — ball and socket, as well as femoral stem — is replaced. Partial hip replacement requires only the ball (femoral head) to be replaced. In a hip resurfacing procedure, the cup is replaced, but the ball is not. Instead, the ball is reshaped and covered with a metal cap.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog



 Problems with transvaginal mesh systems marketed by a number of different medical device manufacturers have caused women throughout the United States to suffer severe injuries.
Problems with transvaginal mesh systems marketed by a number of different medical device manufacturers have caused women throughout the United States to suffer severe injuries.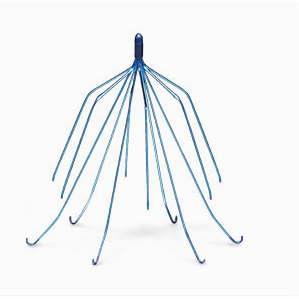 A device known as a vena cava filter (IVC) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. Three devices manufactured by C.R. Bard quickly came under criticism after reports surfaced of complications.
A device known as a vena cava filter (IVC) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. Three devices manufactured by C.R. Bard quickly came under criticism after reports surfaced of complications. While some in the medical community continue to debate whether or not Talcum Powder, also known as Baby Powder, may cause ovarian cancer, two recent lawsuits suggest it does cause ovarian cancer.
While some in the medical community continue to debate whether or not Talcum Powder, also known as Baby Powder, may cause ovarian cancer, two recent lawsuits suggest it does cause ovarian cancer.