Cancer patients receiving chemotherapy understand there is a risk of hair loss involved. In fact, along with nausea, hair loss is the most common and well known side effect of cancer drugs. Cancer paitents tolerate hair loss as a side effect because of the hope that the drug will save their lives and the knowledge that their hair will grow back after cancer treatment.
But a growing number of lawsuits filed against the makers of Taxotere allege that the company failed to warn patients that Taxotere may cause permanent hair loss. The lawsuits also claim that the drug manufacturer actively encouraged doctors to use Taxotere despite its risks and despite the availability of other, safer cancer treatments.
Taxotere was first approved by the FDA in 1996. It was a more powerful version of another chemotherapy drug that had already been approved for use. Taxotere has been approved for the treatment of metastatic prostate cancer, non-small cell lung cancer, neck and head cancer, advance stomach cancer and breast cancer. It is also being investigated as a possible treatment for other cancers, including small cell lung cancer and pancreatic cancer.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog



 The death of a love one is always a difficult situation, but it is especially difficult when someone dies as a result of the negligence or wrongful act of another person. In these situations the next of ken or lawful representative of the estate may file a wrongful death lawsuit against the negligent party.
The death of a love one is always a difficult situation, but it is especially difficult when someone dies as a result of the negligence or wrongful act of another person. In these situations the next of ken or lawful representative of the estate may file a wrongful death lawsuit against the negligent party.  CVS stores are one of the most popular stores in America, and its large volume of customers means that, inevitably, injuries occur on store premises.
CVS stores are one of the most popular stores in America, and its large volume of customers means that, inevitably, injuries occur on store premises. Recent lawsuits against Johnson and Johnson’s Baby Powder product has brought to light evidence linking Talc, the main ingredient in Baby Powder, and ovarian cancer.
Recent lawsuits against Johnson and Johnson’s Baby Powder product has brought to light evidence linking Talc, the main ingredient in Baby Powder, and ovarian cancer.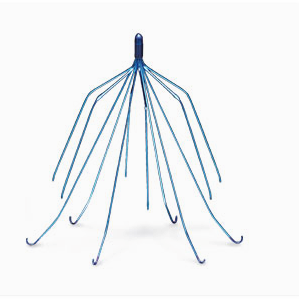 Cook Medical, the makers of Gunther Tulip and other IVC filters, continue to face lawsuits from consumers who have accused the manufacturer of “selling dangerous medical devices and failing to warn about the risks” consumers face.
Cook Medical, the makers of Gunther Tulip and other IVC filters, continue to face lawsuits from consumers who have accused the manufacturer of “selling dangerous medical devices and failing to warn about the risks” consumers face. More than a decade after the first injury linked to Takata’s defective air bags occurred, the largest recall in U.S. automobile history is underway. An estimated 100 million vehicles across the world are being recalled altogether.
More than a decade after the first injury linked to Takata’s defective air bags occurred, the largest recall in U.S. automobile history is underway. An estimated 100 million vehicles across the world are being recalled altogether. Whenever you’re involved in an accident in which one of the driver was driving a motorcycle, the risk of major injuries significantly increase. There might also be some complicated legal issues involved that require an experienced attorney.
Whenever you’re involved in an accident in which one of the driver was driving a motorcycle, the risk of major injuries significantly increase. There might also be some complicated legal issues involved that require an experienced attorney.