Case in point, Amber Nordin, from South Dakota earlier this year filed a personal injury and defective product liability lawsuit against C.R. Bard, because of the Bard company’s Eclipse blood clot filters broke apart and embedded inside her internal organs.
Dr Shezad Malik Law Firm has offices based in Fort Worth and Dallas and represents people who have suffered catastrophic and serious personal injuries including wrongful death, caused by the negligence or recklessness of others. We specialize in Personal Injury trial litigation and focus our energy and efforts on those we represent.
Xarelto Bleeding Side Effect Death Lawsuits Increasing
As a Dallas Xarelto Bleeding Death attorney and Texas medical doctor, I would like to update my readers about the ongoing status of the Xarelto bleeding side effects and wrongful death litigation. Most recently, a wrongful death lawsuit was filed against the makers of Xarelto; Bayer Healthcare and Johnson and Johnson’s subsidiary, Janssen.
Talcum Powder Use & Ovarian Cancer
 Fox died from ovarian cancer in October 2015. In February 2015, a jury in St. Louis found that talcum powder caused the ovarian cancer disease and that J&J was liable for negligence, conspiracy, and failure to warn women of the risk of using talcum Baby Powder in the genital area.
Fox died from ovarian cancer in October 2015. In February 2015, a jury in St. Louis found that talcum powder caused the ovarian cancer disease and that J&J was liable for negligence, conspiracy, and failure to warn women of the risk of using talcum Baby Powder in the genital area.
Talcum Baby Powder Lawsuits
Xarelto Bleeding Side Effects Study Flawed
According to recent revelations in the New York Times did two major pharmaceutical companies, to protect their blockbuster drug, Xarelto, intentionally mislead editors at one of the world’s most prestigious medical journals?
Several thousand injured plaintiffs have filed personal injury and product liability claims against Johnson & Johnson and Bayer over the safety of its anti-clotting drug Xarelto and its increased risk of potentially deadly bleeding side effects.
Now plaintiffs claim that a letter published in The New England Journal of Medicine and written by researchers at Duke University deliberately left out critical laboratory data. They claim the companies were complicit by staying silent, helping deceive the editors while the companies provided the very same data to regulators in the United States and Europe.
The New York Times article suggests that Bayer, Johnson & Johnson and those who ran clinical trials at Duke University that led to the FDA approval of the blood thinner, may have lied to editors at the New England Journal of Medicine.
Defective Medical Device Results In Flawed Xarelto data
The heart of the controversy alleges that a key medical device that measures the levels of blood thinner in patients involved in the study was defective and that those running the clinical trial knew it, but failed to reveal that information.
The New York Times reports that documents produced by the drug makers during Xarelto lawsuits suggest that those running the clinical trial were asked if there were lab tests that confirmed the accuracy of the device. The editors were told there was not, when in actuality there were such tests.
It is now confirmed that the measuring device was defective and may have compromised the approval process for Xarelto, which has since been promoted as a superior alternative to warfarin. Warfarin is the gold standard anticoagulant drug that has been in use for the past 60 years.
Flawed Xarelto Bleeding Medical Studies?
The Xarelto blood testing problems in the clinical trial were first reported in the medical journal The BMJ in December, with researchers warning that the device may have led to an underestimation of the rate of Xarelto bleeding complications in comparison to warfarin.
The ROCKET-AF clinical trials compared the rate of bleeding events between Xarelto and warfarin. The potentially defective blood testing device, known as the INRatio by Alere, was used to measure the levels of warfarin in patients’ blood and was used to adjust their dosage. An INRatio recall has since been issued after it was discovered that the device may show results that were falsely low.
The recall could affect the ROCKET-AF results, because falsely low readings may have resulted in warfarin patients being given too high a dose, increasing their risk of bleeding. If the device caused excessive bleeding among warfarin patients, it could have given the false impression that Xarelto had a lower rate of bleeding problems.
The clinical trials, led by Dr. Robert Califf, who is now the FDA commissioner, have come under intense criticism since Xarelto was approved, as the drug has been linked to a shocking number of adverse event reports involving severe and uncontrollable bleeding problems. Due to a lack of a reversal agent for Xarelto, doctors have been unable to stop serious bleeding problems that occur, increasing the risk of severe injury or death.
Xarelto Lawsuits Over Bleeding Problems
Xarelto is a new class of blood thinners released in recent years as a replacement for warfarin. Xarelto was approved in 2011, this new-generation treatment has been prescribed instead of warfarin to reduce the risk of blood clots and strokes among patients with atrial fibrillation, or following hip or knee replacement surgery.
Xarelto lawsuits allege that the drug makers provided false and misleading information about the importance of blood monitoring on Xarelto, marketing the drug as easier to use and indicating that it does not require close testing like warfarin. But, independent studies published after Xarelto was introduced have suggested that Xarelto monitoring may help identify patients at greater risk of bleeds.
Undue Big Pharma Influence for Xarelto
Big Pharma is the nickname given to the vast and influential pharmaceutical industry and its trade group, the Pharmaceutical Research and Manufacturers of America or PhRMA. These powerful companies make billions of dollars every year by selling drugs and medical devices.
Pharmaceuticals are HUGE business and these Big Pharma” companies stand to reap billions of dollars over the life span of a block buster drug. Big Pharma industry influence has led to the concealment of critical unfavorable data or ghost written medical articles (written by industry insiders) — when crucial clinical data went missing from journal articles, leading to embarrassing corrections and ethics policies to limit the influence of drug companies on medical literature.
Xarelto Billion Dollar Block Buster
Xarelto, is sold in the United States by Johnson & Johnson and overseas by Bayer, had nearly $2 billion in United States sales last year. Xarelto is the best seller in a new category of drugs seeking to replace warfarin.
Recently, lawyers in the case against Johnson & Johnson and Bayer filed in federal court in New Orleans, have asked the judge to unseal documents in the case, which involves more than 5,000 lawsuits filed by patients and their families who claim they were harmed by Xarelto. Of those, 500 involve patient deaths.
Dr. Steven Nissen, a cardiologist at the Cleveland Clinic, served on the Food and Drug Administration advisory panel that voted to approve Xarelto in 2011. He was one of two members who voted against the drug. He expressed doubt that any after-the-fact analysis would give doctors and patients answers. “Given the fact that the device was inaccurate, there is no way anybody can tell you what would have happened in the trial,” he said.
DePuy Pinnacle Hip Lawsuit $502 M Verdict
This is an update to the DePuy Pinnacle Hip lawsuits. DePuy Pinnacle Hip implants have a tendency to fail prematurely, cause hip infections, elevated heavy metal ions and poisoning. Recently, a Texas federal jury in Dallas has awarded $502 million total to five separate plaintiffs after a lengthy consolidated trial involving defective DePuy Pinnacle Hip Implants manufactured by DePuy Orthopaedics and Johnson & Johnson (J&J).
Johnson and Johnson and its subsidiary DePuy is under fire for premature failure of its line of metal on metal hip implants.
DePuy Orthopaedics and Johnson & Johnson was found liable hiding flaws in its DePuy Pinnacle line of metal on metal artificial hips that caused the devices to fail prematurely and left them facing revision surgeries and pain.
The landmark verdict delivered today follows claims filed by Margaret Aoki, Jay Christopher, Donald Greer, Richard Klusmann and Robert Peterson, all Texas residents who received DePuy’s Pinnacle Acetabular Cup System hip implant.
All five alleged that the devices had unreasonably high failure rates resulting in severe pain and inflammation, bone erosion, tissue loss and other problems.
The hip implant victims claimed that DePuy’s hip design was defective; that the company failed to give adequate warnings; and Johnson & Johnson aided and abetted in misrepresentations that rose to the level of fraud.
They awarded $142 million in actual damages and $360 million in punitive damages to a group of five patients whose hips broke down and had to be surgically removed.
The U.S. District Court for the Northern District of Texas jury announced the verdict on March 17 after hearing 37 days of testimony.
J&J DePuy Pinnacle Hip Implant Failures
Johnson & Johnson’s DePuy Orthopaedics subsidiary continues to face thousands of lawsuits over their defective metal-on-metal Pinnacle hip replacement. The Pinnacle metal-on-metal hip implant system was manufactured from 2002 to 2012 before DePuy pulled the metal liners off the market in 2013.
The Pinnacle devices have never been recalled, although DePuy recalled other metal-on-metal hip implants that were sold under the ASR brand name to roughly 35,000 patients here in the U.S.
According to the latest court data from March 15, 2016, there are more than 8,300 federal product liability lawsuits that have been filed by folks who experienced severe and serious problems with the artificial hip implant.
DePuy Pinnacle Hip Lawsuit Multidistrict Litigation
Since 2011 all federal DePuy Pinnacle cases has been consolidated and centralized in the U.S. District Court for the Northern District of Texas, as part of an MDL, or Multidistrict Litigation.
Over 8,300 similar claims have been centralized in the Northern District of Texas before U.S. District Judge Ed Kinkeade to streamline the discovery and bellwether trial process. The consolidated for pretrial proceedings were established in May 2011.
The first bellwether trial in 2014, found for the defense when the jury found that the patient injuries may have been as a result of poor doctor implant techniques.
DePuy Pinnacle Hip Failure
There are thousands of DePuy Pinnacle hip lawsuits that allege that the DePuy Pinnacle hip has an unreasonably dangerous and defective design, which was not thoroughly studied and that inadequate warnings were provided about the risk that the device may loosen and fail.
Microscopic metal particles are released into the body from the metal components, which are made from Cobalt and Chromium alloy grinding against each other during normal daily activities. This release of cobalt and chromium particles into the body is known as metallosis.
The jury found that DePuy took a number of short cuts getting the Pinnacle hip implants on the market, in part due to the 510(k) approval process, which only requires a device be substantially equivalent to devices already in use.
The company did not seek premarket approval, and so avoided the FDA requirement to determine if a device is safe or effective. There were no Pinnacle hip clinical trials to see if it even worked, or if it was safe to use.
The FDA released new guidance for metal-on-metal hip replacements in January 2013, indicating that doctors should only use the design if other artificial hip implants are not appropriate. The agency also determined that future metal-on-metal hip designs will be required to undergo extensive human clinical trials before they will be approved.
Taxotere Baldness from Chemotherapy
Chemotherapy can cause permanent hair loss especially in the treatment of breast cancer . This hair loss can be permanent and devastating. Sanofi-Aventis, the maker of a popular intravenous chemotherapy drug, Taxotere, is under fire in several lawsuits claiming that Taxotere, may cause permanent hair loss or baldness (Alopecia) in women.
Recently, Hattie Carson filed her claim of Taxotere baldness from chemotherapy side effects, in the U.S. District Court for the Northern District of Ohio. Carson, claims that Sanofi-Aventis failed to adequately warn female breast cancer patients and doctors about the risk of Taxotere permanent baldness.
According to Carson, she was diagnosed with breast cancer in 2004, and underwent Taxotere chemotherapy. Carson was diagnosed with permanent hair loss, after her hair did not grow back after 6 months.
Taxotere Baldness
Baldness is a common and emotionally devastating side-effect of chemotherapy. Scalp, pubic and axillary hair may be lost, as well as eyebrows and eyelashes, but in most cases, the hair loss is temporary and usually reversible, with complete hair regrowth within the 3–6 months following the chemotherapy cycles.
Recently, in the past few years, many case reports of permanent, irreversible post-chemotherapy baldness, no hair growth after 6 months have been described from Taxotere.
What is Taxotere?
Taxotere (docetaxel) is a cancer medication that interferes with the growth and spread of cancer cells in the body. Taxotere is used to treat breast cancer, lung cancer, prostate cancer, stomach cancer, and head/neck cancer.
Taxotere is a cancer drug given in a cocktail of chemotherapy drugs, for the treatment of breast cancer. Taxotere was approved by the FDA in May 1996 for the treatment of patients with advanced or metastatic breast cancer after prior chemotherapy attempts had failed.
Carson claims in her lawsuit, “Although alopecia is a common side effect related to chemotherapy drugs, permanent alopecia is not. Defendants, through its publications and marketing material, misled Plaintiff, the public, and the medical community to believe that, as with other chemotherapy drugs that cause alopecia, patients’ hair would grow back.”
Sanofi-Aventis Warned About Taxotere Baldness
Carson alleges that Sanofi-Aventis warned doctors and patients in Europe in 2005 and in Canada 2012 about the risks of permanent hair loss from Taxotere side effects, but failed to provide such warnings to the U.S. patients and doctors until January 2016. Read the FDA Warning label change in January 2016.
Taxotere Baldness “Disfiguring”
According to Carson, Taxotere baldness is disfiguring for women, and that it caused her to suffer great mental anguish, economic damages and that the psychological damage was extensive enough that it caused a loss of work or inability to work.
Taxotere “I look like an 80-year-old, ugly old man”
Taxotere baldness injured women are now warning other permanent hair loss victims in social media venues. According to a 2010 article in the Globe and Mail, “I had a normal head of hair and I am now completely bald,” said Cynthia MacGregor, 50, of Montreal, who has been diagnosed with alopecia universalis, a loss of all body hair.
According to Shirley Ledlie, 51, of Brittany, France, said: “It’s like having ‘I am a cancer sufferer’ tattooed on your forehead. … I look like an 80-year-old, ugly old man.”
Taxotere affected permanent balding women from Canada, the United States, the United Kingdom and France are calling themselves the Taxotears. They include one Taxoterrorist, the nickname for Ms. Ledlie, who posted pictures of her balding head on the Facebook page of the pharmaceutical company.
“We want every woman who’s been offered Taxotere to know it is a possibility, so it is her choice whether to take the risk or not,” Ms. Ledlie said.
Abilify Lawsuits
Abilify lawsuits are now being filed nationwide alleging economic losses from Abilify. Bristol-Myers Squibb and Otsuka Pharmaceutical Co., the makers of Abilify are under fire for the side effects of their blockbuster drug. The companies are exposed to a new product liability lawsuit, which claims that the compulsive or pathological gambling side effects associated with the popular antipsychotic medication causes severe financial harm.
Nicholas Meyer recently filed against Bristol-Myers Squibb and Otsuka Pharmaceutical Co. in the U.S. District Court for the Southern District of Indiana.
Meyer alleges that he suffered Abilify gambling losses in excess of $45,000 after starting to use Abilify. According to Meyer, he began using the drug in November 2010, and developed a compulsive gambling habit.
Meyer claims that the compulsive gambling problems stopped once he stopped taking the drug, but he has been left with economic damages from the side effects of Abilify.
Meyer in his Abilify lawsuit claims that, “The injurious impact of Abilify on the Plaintiff’s brain constitutes a physical injury, and as a result of Abilify use, Plaintiff has suffered, and will continue to suffer, neuro-psychiatric and physical injury, emotional distress, harm, and economic loss as alleged herein.”
Abilify Compulsive Gambling Problems
Abilify (ariprazole) is a blockbuster cash cow drug for Bristol-Myers Squibb and Otsuka Pharmaceutical Co., in the United States, with sales in excess of $6 billion per year.
Abilify was approved by the FDA in 2002 for treatment of schizophrenia, bipolar disorder and other major depressive disorders, but is also widely used off-label to treat irritability, aggression, mood swings and other behavioral issues including autism spectrum disorders.
Abilify Warnings in Europe and Canada
In November 2012, European drug regulators required Abilify pathological gambling warnings, adding information to the label about reports of users engaging in uncontrollable gambling activities and recommending that patients should be monitored carefully. But the companies neglected to provide similar warnings to patients and doctors in the United States.
The companies provided warnings about the link between Abilify and gambling to the Canadian doctors in November 2015, after governmental regulators found cases where patients began compulsive gambling or hypersexuality behavior.
Abilify Lawsuits
Several Abilify gambling lawsuits have been filed in recent months throughout the United States, claiming that the drug manufacturers knew or should have known that the Abilify patients face an increased risk of uncontrollable gambling.
The plaintiffs blame the makers for withholding warnings from patients and doctors that may have allowed folks to identify the problems before they suffered damages to their financial stability.
Medical Studies: Abilify and Gambling
A study published in October 2014 in JAMA Internal Medicine found that medications known as dopamine receptor agonists, that are used to treat Parkinson’s disease, were associated with impulse control problems. The researchers also found the same abnormal behavioral problems with the use of Abilify.
Abilify has been linked to compulsive behavior side effects, such as pathological gambling, binge eating and hypersexuality. These behaviors are triggered by the neurotransmitters dopamine and serotonin.
When the dopamine system is stimulated in response to a particular activity, people will feel a high from it or a feeling of pleasure. Medical experts think Abilify may over-stimulate dopamine reward receptors in the brain – called dopamine 3 (D3) receptors – and trigger compulsive behavior.
Abilify Lawsuits: Devastating Financial Losses
Compulsive addictive gambling from Abilify can have a tremendously devastating impact on patients, causing serious financial losses and reckless behavior that can destroy families, and cause irreversible damage folks. When a person has a compulsive symptom, a brain irregularity makes some behaviors impossible to stop without medical assistance.
Compulsive gamblers have a tough time walking away from gambling facilities including slot machines, blackjack tables, casinos, roulette wheels, horse and greyhound racing, and poker tournaments even if they are broke. One sad and tragic outcome is that they gamble on credit and drive themselves into unsustainable debt, ruining their and their families lives.
Plaintiffs claim if they were adequately warned about the risk of compulsive behaviors, they may have avoided the damaging consequences by stopping use of Abilify.
Bad Things Can Happen With Bard IVC Filters
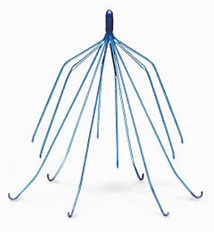
South Dakota Bard IVC Filter Injury
Amber Nordin, from South Dakota earlier this year filed a personal injury and defective product liability lawsuit against C.R. Bard, because of the company’s Eclipse blood clot filters broke apart and embedded inside her internal organs.
Laparoscopic Hysterectomy Uterine Cancer Lawsuit Battle
 Davis has to take expensive oral chemotherapy drugs to keep the leiomyosarcoma cancer from spreading further and she knows that beating the uterine cancer is going to be a tough battle that she may not win.
Davis has to take expensive oral chemotherapy drugs to keep the leiomyosarcoma cancer from spreading further and she knows that beating the uterine cancer is going to be a tough battle that she may not win.
Increased Risk of Cancer Spread After Morcellator Use
Talcum Powder Ovarian Cancer Victims
Medical experts have warned that women who regularly powder their genitals with talc have a one-third higher risk of being diagnosed with ovarian cancer.
 Those women who routinely apply talc to their genitals, sanitary napkins, tampons and underwear were found to have a 33 per cent higher risk of ovarian cancer.
Those women who routinely apply talc to their genitals, sanitary napkins, tampons and underwear were found to have a 33 per cent higher risk of ovarian cancer.
Lead study author Dr Daniel Cramer, of Brigham and Women’s Hospital in Boston, noted that there must be warning labels on talcum powder.
According to Cramer, “Talc is a good drying agent, but women should know that if it’s used repeatedly, it can get into the vagina and into their upper genital tract.”
Dr Cramer first linked genital talc to ovarian cancer in 1982. The current study, in the journal Epidemiology, is the first to limit the association to premenopausal women and postmenopausal women who used hormone therapy.
What is Talc?
Talc is a naturally occurring mineral that absorbs moisture, and is made of magnesium, silicon and oxygen. It may contain asbestos – a known carcinogen – in its natural form. But, all consumer talc products in the US have been free of asbestos in the US since the 1970s.
Ovarian Cancer
Almost 20,000 women in the US are diagnosed with ovarian cancer each year – and almost 14,500 die from the disease annually, according to the Centers for Disease Control and Prevention (CDC).
The World Health Organization’s International Agency for Research on Cancer classified genital talc as possibly carcinogenic in 2006.
St Louis Talc Powder Ovarian Cancer $72 Million Verdict
The St. Louis jury in the latest J&J talc powder lawsuit to go to trial, was hammered and ordered to pay $72 million to the family of a woman who died from ovarian cancer. The family of Jackie Fox filed a wrongful death claim for ovarian cancer caused by talcum powder that she used throughout her life.
Fox used J&J talcum powder products for feminine hygiene, and she alleged that the company failed to warn that the talc migrates through the vagina and increase the risk of ovarian cancer.
$62 Million In Punitive Damages
The jury in the Missouri Circuit Court in St. Louis, found that J&J should pay the family $10 million in compensatory damages, for causing her wrongful death. The jury also found that J&J acted with gross negligence and wanton disregard for the plaintiff’s health and safety, and awarded $62 million in punitive damages. Punitive damages are awarded when the jury finds egregious behavior and is designed to punish the company for wrongful acts.
The jury noted that internal documents showed that the company knew about increased risk of ovarian cancer from talc for years, but hid the vital information from talc powder users.
Smoking Gun Internal Documents
According to the jury the company’s internal documents that were presented during the trial, swayed the jury towards for finding for the plaintiffs. A 1997 internal memo from a company medical consultant said “anybody who denies” the risk of using hygienic talc and ovarian cancer is “denying the obvious in the face of all evidence to the contrary,” AP reported.
Shower-to-Shower and Baby Powder Under Fire
Johnson’s Baby Powder is the most popular talcum powder product sold by the company. The talc is usually used in infants to prevent diaper rashes and it is also marketed to adult women for general hygiene purposes, as the Shower-to-Shower product.
Johnson & Johnson, based in New Brunswick, N.J., is expected to appeal. It issued a statement Tuesday insisting the products are safe.
1,200 Talc Ovarian Cancer Lawsuits Pending
There are about 1,200 Baby Powder lawsuits and Shower-to-Shower body powder lawsuits pending against J&J, with similar allegations that the company failed to warn that the talcum-based powders may increase the risk of ovarian cancer when applied to the female genitals.
According to medical experts, pathological evidence reveals that the talc may migrate through the vagina and into the fallopian tubes, uterus and ovaries, causing inflammation leading ovarian cancers. Microscopic evidence of talc particulate infiltration is found in the ovaries of affected women.
2nd Jury Talc Ovarian Cancer Verdict
In October 2013, a South Dakota jury found that J&J failed to warn about the risk of ovarian cancer from talcum powder, but no damages were awarded. In that South Dakota case, Deane Berg, aged 56 was diagnosed with ovarian cancer, and three doctors found that her cancer tissue had evidence of talc in the ovaries.
In fall 2006, Berg began noticing signs of ovarian cancer. Berg had both ovaries removed when her doctors found a hemorrhagic ovary. In January 2007 Berg was diagnosed with stage 3 ovarian cancer with a life expectancy of less than five years. Berg’s oncologist suggested a linkage between talcum powder and ovarian cancer. Berg had “dusted” her groin daily with J&J’s talcum powder since the age of 18.
New Jersey Talc Powder State Court Mass Tort
The New Jersey Supreme Court in November 2015, consolidated all talcum powder lawsuits filed on behalf of women diagnosed with ovarian cancer.
All talc cancer cases filed throughout New Jersey state court are centralized before Judges Julio L. Mendez and Nelson C. Johnson in Atlantic County, as part of a Multi-County Litigation (MCL). There are more than 100 ovarian cancer lawsuits over talcum powder pending throughout the state.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog


