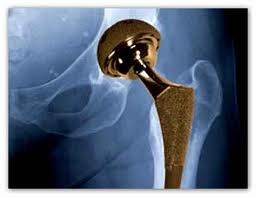
Stryker, a well known artificial hip and medical device manufacturer, is recalling devices used in spinal surgeries. The FDA has assigned its most serious warning label to the problem, indicating that using the device could result in serious injury or death. Stryker is already under fire over lawsuits involving its Stryker Rejuvenate and ABG II artificial hips, amid allegations of early failure of the devices and the risk of metallosis. Metallosis occurs when the component parts grind against each other releasing metal ions into the bloodstream and hip joint, leading to loosening of the hip device, bone destruction, pain and swelling.
 The device is the Oasys Occipito-Cervico-Thoracic System, which is designed to stabilize the junction between the occipital bone and vertebrae in the cervical spine.
The device is the Oasys Occipito-Cervico-Thoracic System, which is designed to stabilize the junction between the occipital bone and vertebrae in the cervical spine.
Stryker has received reports that a pin that connects the tulip head to the plate body can fracture, possibly causing blood loss, nerve injury and the need for revision surgery. The FDA put its Class I designation on the recall, a notification for potentially deadly malfunctions.

 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog